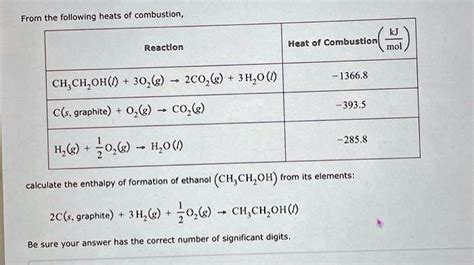
Enthalpy Change for Combustion of Ethanol: -1366.8 kJ/mol
Introduction
Ethanol is a renewable fuel that is produced from the fermentation of sugars. It is a clean-burning fuel that produces less pollution than gasoline. The combustion of ethanol releases energy, which can be used to power engines or generate electricity.

The enthalpy change for the combustion of ethanol is -1366.8 kJ/mol. This means that when one mole of ethanol is burned, 1366.8 kJ of energy is released. This energy can be used to do work, such as powering an engine or generating electricity.
Applications of Ethanol Combustion
Ethanol is a versatile fuel that can be used in a variety of applications. Some of the most common applications include:
- Transportation: Ethanol can be used as a fuel for cars, trucks, and buses. It can also be used as a blend with gasoline.
- Power generation: Ethanol can be used to generate electricity. Ethanol-powered power plants are a clean and renewable source of energy.
- Heating: Ethanol can be used to heat homes and businesses. Ethanol-powered furnaces and boilers are a clean and efficient way to heat buildings.
- Industrial: Ethanol can be used as a solvent and a cleaning agent. It is also used in the production of chemicals and pharmaceuticals.
Benefits of Ethanol Combustion
Ethanol combustion has a number of benefits over other fuels, including:
- Reduced pollution: Ethanol produces less pollution than gasoline when burned. This is because ethanol is a cleaner-burning fuel that produces fewer emissions.
- Renewable resource: Ethanol is a renewable resource that is produced from plants. This makes it a sustainable fuel source that can be used to reduce our dependence on fossil fuels.
- Cost-effective: Ethanol is a cost-effective fuel that is comparable in price to gasoline. This makes it a good option for consumers who are looking to save money on fuel costs.
How to Use Ethanol Combustion Safely
Ethanol combustion is a safe process when done properly. However, there are some precautions that should be taken when using ethanol as a fuel.
- Store ethanol in a safe place: Ethanol should be stored in a cool, dry place away from heat and open flames.
- Use ethanol in a well-ventilated area: Ethanol should be used in a well-ventilated area to avoid the buildup of fumes.
- Do not smoke or use open flames near ethanol: Ethanol is flammable, so it is important to avoid smoking or using open flames near it.
Common Mistakes to Avoid
There are a few common mistakes that people make when using ethanol combustion. These mistakes can be dangerous, so it is important to avoid them.
- Using ethanol in an unapproved appliance: Ethanol should only be used in appliances that are approved for ethanol use. Using ethanol in an unapproved appliance can be dangerous.
- Overfilling the fuel tank: Do not overfill the fuel tank on an ethanol-powered appliance. Overfilling the fuel tank can cause the appliance to malfunction.
- Spilling ethanol: If you spill ethanol, clean it up immediately. Ethanol is flammable, so it is important to avoid spills.
Step-by-Step Approach to Ethanol Combustion
The following is a step-by-step approach to ethanol combustion:
- Store ethanol in a safe place: Ethanol should be stored in a cool, dry place away from heat and open flames.
- Use ethanol in a well-ventilated area: Ethanol should be used in a well-ventilated area to avoid the buildup of fumes.
- Fill the fuel tank: Fill the fuel tank on the ethanol-powered appliance to the appropriate level.
- Light the appliance: Follow the manufacturer’s instructions for lighting the appliance.
- Monitor the appliance: Monitor the appliance while it is running to ensure that it is functioning properly.
FAQs
1. What is the enthalpy change for the combustion of ethanol?
The enthalpy change for the combustion of ethanol is -1366.8 kJ/mol.
2. What are the benefits of ethanol combustion?
The benefits of ethanol combustion include reduced pollution, renewable resource, and cost-effective.
3. How to use ethanol combustion safely?
Ethanol combustion is a safe process when done properly. However, there are some precautions that should be taken when using ethanol as a fuel.
4. What are the common mistakes to avoid when using ethanol combustion?
The common mistakes to avoid when using ethanol combustion include using ethanol in an unapproved appliance, overfilling the fuel tank, and spilling ethanol.
Conclusion
Ethanol combustion is a clean, renewable, and cost-effective way to produce energy. Ethanol can be used in a variety of applications, including transportation, power generation, heating, and industrial. Ethanol combustion is a safe process when done properly. However, there are some precautions that should be taken when using ethanol as a fuel.