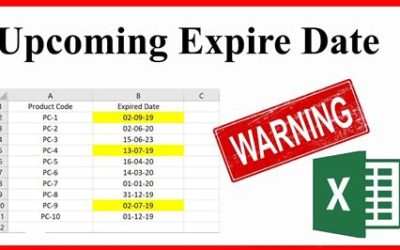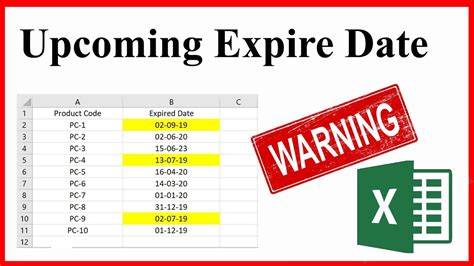
17 Key Differences Between Addition and Condensation Polymerization
Introduction to Polymerization
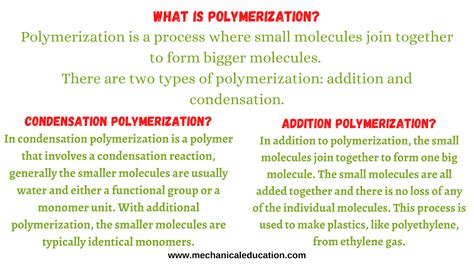
Polymerization is a chemical process where small molecules (monomers) connect to form much larger molecules (polymers). It’s classified into two major types: addition polymerization and condensation polymerization, which differ significantly in their mechanisms, monomers, and properties.

1. Monomer Structure
- Addition Polymerization: Monomers usually have double or triple carbon-carbon bonds (alkenes, alkynes).
- Condensation Polymerization: Monomers contain functional groups, such as hydroxyl (-OH), carboxylic acid (-COOH), or amine (-NH2), that can undergo condensation reactions.
2. Reaction Mechanism
- Addition Polymerization: Monomers join by adding onto the growing polymer chain through a free radical or ionic mechanism.
- Condensation Polymerization: Monomers react with each other to form covalent bonds, releasing a small molecule like water or alcohol.
3. Molecular Structure
- Addition Polymerization: Polymers have a regular, linear or branched structure, depending on the monomer and polymerization conditions.
- Condensation Polymerization: Polymers can have complex, branched or cross-linked structures due to multifunctional monomers.
4. Monomer Functionality
- Addition Polymerization: Monomers are monofunctional, having one reactive double or triple bond per molecule.
- Condensation Polymerization: Monomers are bifunctional or polyfunctional, containing two or more reactive functional groups per molecule.
5. Initiation
- Addition Polymerization: Initiated by free radicals or ions, which react with monomers to start chain growth.
- Condensation Polymerization: Often initiated by heat or acid catalysts that activate functional groups for condensation reactions.
6. Termination
- Addition Polymerization: Chain growth terminates when two active polymer chains combine or when they react with inhibitors.
- Condensation Polymerization: Reaction continues until functional groups are exhausted or until the polymer reaches a desired molecular weight.
7. Water Tolerance
- Addition Polymerization: Typically not water-tolerant, as water can interfere with the free radical or ionic mechanisms.
- Condensation Polymerization: Can be water-tolerant, as condensation reactions don’t require free radicals or ions.
8. Applications
- Addition Polymerization: Polyethylene (PE), polypropylene (PP), polyvinyl chloride (PVC), polystyrene (PS)
- Condensation Polymerization: Polyester (PET), nylon, polycarbonate (PC), polyurethane (PU)
9. Properties
- Addition Polymers: Generally have high strength, toughness, and elasticity.
- Condensation Polymers: Often possess strong intermolecular forces, leading to higher rigidity, thermal stability, and chemical resistance.
10. Processing
- Addition Polymers: Can be processed by melt extrusion or injection molding.
- Condensation Polymers: Often require curing or cross-linking steps to achieve desirable properties.
11. Thermal Stability
- Addition Polymers: Generally more thermally stable, with higher glass transition temperatures (Tg).
- Condensation Polymers: May have lower Tg due to weaker intermolecular forces, but can be cross-linked to improve thermal stability.
12. Biodegradability
- Addition Polymers: Typically non-biodegradable, due to their inert carbon-carbon bonds.
- Condensation Polymers: Some can be biodegradable, as they contain hydrolyzable bonds (e.g., polyesters, aliphatic nylons).
13. Solubility
- Addition Polymers: Generally insoluble in water, due to their nonpolar nature.
- Condensation Polymers: Can be hydrophilic or hydrophobic, depending on the functional groups present.
14. Applications in Biomaterials
- Addition Polymers: Used in medical implants, catheters, and tissue engineering scaffolds.
- Condensation Polymers: Employed in drug delivery systems, biocompatible coatings, and regenerative medicine.
15. Size and Shape Control
- Addition Polymerization: Monomer addition provides precise control over polymer size and shape.
- Condensation Polymerization: Monomer functionality and reaction conditions can influence polymer length and branching.
16. Nanostructures and Functionalization
- Addition Polymers: Can be modified with nanostructures or functional groups through copolymerization or post-polymerization reactions.
- Condensation Polymers: Can be tailored with various функциональные группы and can form complex, self-assembled structures.
17. Environmental Considerations
- Addition Polymers: Can contribute to plastic waste and pollution due to their non-biodegradability.
- Condensation Polymers: Some are biodegradable and can offer sustainable alternatives to traditional plastics.
Conclusion
Understanding the key differences between addition and condensation polymerization is crucial for selecting appropriate materials and tailoring polymer properties for specific applications. As research continues, novel polymerization techniques and innovative polymer materials with tailored functionalities and properties are on the horizon, opening up new possibilities in various industries.