Dissociation of Water: Exploring the 1.8 x 10^-16 Ion Product
Introduction
Water, the elixir of life, plays a crucial role in countless chemical reactions and biological processes. One of its fundamental properties is its ability to undergo dissociation, forming ions that influence the pH and electrical conductivity of aqueous solutions. This article delves deep into the fascinating world of water dissociation and its ion product, providing a comprehensive understanding of this essential phenomenon.

The Dissociation of Water
In pure water, a small fraction of water molecules undergo dissociation, resulting in the formation of hydrogen ions (H+) and hydroxide ions (OH-). This process, known as autoionization, can be represented by the chemical equation:
H2O (l) <=> H+ (aq) + OH- (aq)
At 25°C, the concentration of H+ and OH- ions in pure water is equal to 1.0 x 10^-7 moles per liter (mol/L). This value, known as the dissociation constant (Kw), is a fundamental property of water and reflects the extent to which it dissociates.
The Ion Product
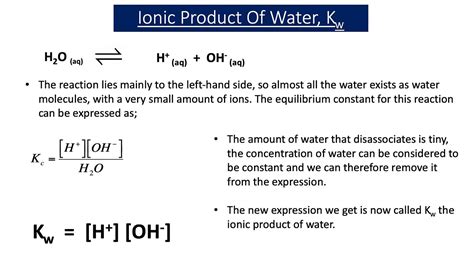
The ion product of water, denoted by Kw, is defined as the product of the molar concentrations of H+ and OH- ions in an aqueous solution. Mathematically, it can be expressed as:
Kw = [H+] x [OH-]
At 25°C, the ion product of water is a constant value of 1.8 x 10^-16. This means that in any aqueous solution, the product of the H+ and OH- ion concentrations will always equal 1.8 x 10^-16.
Factors Affecting Dissociation
The dissociation of water is influenced by several factors, including temperature, ionic strength, and the presence of acids or bases.
Temperature
As temperature increases, the dissociation constant of water increases, indicating that more water molecules dissociate. At 100°C, the Kw value increases to 5.1 x 10^-13.
Ionic Strength
The presence of other ions in an aqueous solution can affect the dissociation of water. High ionic strength, caused by the presence of dissolved salts, suppresses dissociation, leading to lower H+ and OH- ion concentrations.
Acids and Bases
Acids donate H+ ions, while bases donate OH- ions. The addition of acids or bases to an aqueous solution shifts the equilibrium of the dissociation reaction, altering the H+ and OH- ion concentrations.
Applications of Dissociation of Water
The dissociation of water has numerous applications in various fields, including:
1. pH Measurement
The pH of an aqueous solution is a measure of its acidity or basicity. It is directly related to the H+ ion concentration. By understanding the dissociation of water, scientists can develop methods to accurately measure pH using pH meters or indicators.
2. Electrochemistry
The dissociation of water is essential for the functioning of electrochemical cells. In batteries, for example, the dissociation of water produces H+ ions that facilitate the transfer of electrons between electrodes.
3. Water Purification
Distillation and deionization are water purification techniques that rely on the dissociation of water. These processes remove impurities by selectively removing H+ and OH- ions, resulting in pure water with a low ion product.
Tables
Table 1: Dissociation Constants of Water at Different Temperatures
| Temperature (°C) | Dissociation Constant (Kw) |
|---|---|
| 0 | 0.115 x 10^-14 |
| 25 | 1.8 x 10^-16 |
| 50 | 5.47 x 10^-14 |
| 75 | 2.83 x 10^-13 |
| 100 | 5.1 x 10^-13 |
Table 2: Effect of Ionic Strength on Dissociation Constant of Water
| Ionic Strength (mol/kg) | Dissociation Constant (Kw) |
|---|---|
| 0.0 | 1.8 x 10^-16 |
| 0.1 | 1.3 x 10^-16 |
| 0.5 | 0.9 x 10^-16 |
| 1.0 | 0.6 x 10^-16 |
Table 3: Applications of Dissociation of Water
| Application | Description |
|---|---|
| pH Measurement | Determining the acidity or basicity of aqueous solutions |
| Electrochemistry | Facilitating electron transfer in batteries |
| Water Purification | Removing impurities through distillation or deionization |
| Biochemical Processes | Essential for enzyme activity and DNA repair |
Table 4: Factors Affecting Dissociation of Water
| Factor | Effect on Dissociation |
|---|---|
| Temperature | Increases dissociation at higher temperatures |
| Ionic Strength | Suppresses dissociation at higher ionic strengths |
| Acids | Decreases OH- ion concentration |
| Bases | Increases OH- ion concentration |
Conclusion
The dissociation of water, characterized by an ion product of 1.8 x 10^-16, is a fundamental property of water that plays a critical role in numerous chemical and biological processes. Understanding the factors that affect dissociation and its various applications is essential for researchers, scientists, and anyone interested in the fascinating world of water chemistry.