Delta G NFE: What is n?
Introduction
Delta G, also known as Gibbs free energy, is a thermodynamic potential that measures the maximum amount of work that can be extracted from a thermodynamic system at constant temperature and pressure. In the context of redox reactions, Delta G is used to determine the spontaneity and equilibrium of a reaction. The n in Delta G NFE refers to the number of electrons transferred in the reaction.

What is Delta G NFE?
Delta G NFE stands for the standard Gibbs free energy change of a redox reaction. It is the Gibbs free energy change that occurs when one mole of reactants is converted to one mole of products under standard conditions (298 K and 1 atm).
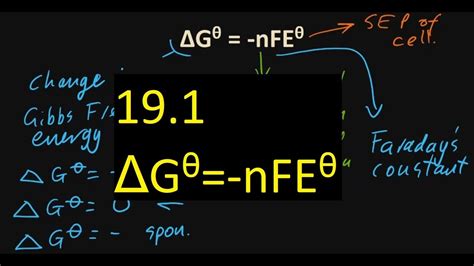
Delta G NFE is calculated using the following equation:
Delta G NFE = -nFE
where:
- n is the number of electrons transferred in the reaction
- F is the Faraday constant (96,485 C/mol)
- E is the standard cell potential
How is Delta G NFE used?
Delta G NFE is used to determine the spontaneity of a redox reaction. A reaction is spontaneous if Delta G NFE is negative. If Delta G NFE is positive, the reaction is non-spontaneous.
Delta G NFE can also be used to determine the equilibrium constant of a redox reaction. The equilibrium constant is a measure of the relative amounts of reactants and products at equilibrium. The equilibrium constant is given by the following equation:
K = e^(-Delta G NFE / RT)
where:
- K is the equilibrium constant
- R is the gas constant (8.314 J/mol*K)
- T is the temperature (K)
Examples of Delta G NFE Calculations
The following are examples of how to calculate Delta G NFE for redox reactions:
Example 1:
Calculate Delta G NFE for the following reaction:
Zn + 2H+ -> Zn2+ + H2
The standard cell potential for this reaction is 0.76 V.
Delta G NFE = -nFE = -(2 * 96,485 C/mol * 0.76 V) = -147,000 J/mol
This negative value of Delta G NFE indicates that the reaction is spontaneous.
Example 2:
Calculate Delta G NFE for the following reaction:
Fe3+ + e- -> Fe2+
The standard cell potential for this reaction is -0.77 V.
Delta G NFE = -nFE = -(1 * 96,485 C/mol * -0.77 V) = 74,400 J/mol
This positive value of Delta G NFE indicates that the reaction is non-spontaneous.
Applications of Delta G NFE
Delta G NFE is used in a variety of applications, including:
- Predicting the spontaneity of redox reactions
- Determining the equilibrium constant of redox reactions
- Designing electrochemical cells
- Developing new energy technologies
Conclusion
Delta G NFE is a powerful tool that can be used to understand and predict the behavior of redox reactions. It is used in a variety of applications, including predicting the spontaneity of reactions, determining the equilibrium constant of reactions, designing electrochemical cells, and developing new energy technologies.