Calculate pH from Ka and Molarity: An In-Depth Guide to 2025
Introduction
Understanding the pH of a solution is crucial in various scientific fields, including chemistry, biology, and environmental science. pH is a measure of the acidity or basicity of a solution and is determined by the concentration of hydrogen ions (H+). In this guide, we will explore the relationship between acid dissociation constant (Ka) and molarity in calculating pH, providing a comprehensive understanding of this fundamental concept.

What is Acid Dissociation Constant (Ka)?
Acid dissociation constant, denoted by Ka, is a quantitative measure of the strength of an acid in aqueous solution. It represents the equilibrium constant for the dissociation of an acid into its conjugate base and hydrogen ions. A higher Ka value indicates a stronger acid.
The Ka expression for a monoprotic acid (HA) is given by:
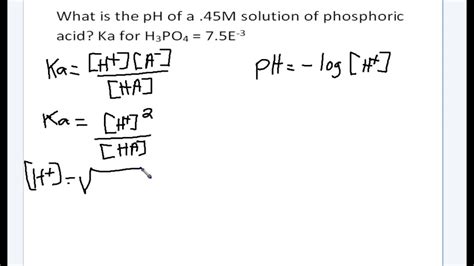
Ka = [H+][A-] / [HA]
where [H+] is the molar concentration of hydrogen ions, [A-] is the molar concentration of the conjugate base, and [HA] is the molar concentration of the undissociated acid.
Molarity and Acid Dissociation
Molarity (M) is a measure of the concentration of a substance in a solution, expressed as the number of moles of the substance per liter of solution. In the context of acid dissociation, molarity plays a significant role in determining the concentration of hydrogen ions in a solution.
When an acid dissociates in water, a certain percentage of its molecules ionize, releasing hydrogen ions. The extent of ionization is directly proportional to the molar concentration of the acid. Higher molarity leads to a higher concentration of hydrogen ions and, consequently, a lower pH.
The Relationship between Ka and Molarity
The relationship between Ka and molarity can be used to calculate the pH of a solution. The following steps outline the process:
-
Determine the Ka value of the acid: Obtain the Ka value from a reference table or calculate it using experimental data.
-
Set up the Ka expression: Write the Ka expression for the acid dissociation reaction.
-
Substitute molarity values: Substitute the molarity of the acid (HA) into the Ka expression.
-
Solve for [H+]: Solve the Ka expression for [H+], the molar concentration of hydrogen ions.
-
Calculate pH: Convert the hydrogen ion concentration to pH using the formula pH = -log[H+].
Practical Applications
Calculating pH from Ka and molarity has numerous applications in diverse fields:
- Chemistry: Determining the pH of acid-base solutions, buffer systems, and chemical reactions.
- Biology: Measuring the pH of biological fluids, such as blood, urine, and saliva.
- Environmental Science: Monitoring the pH of water bodies to assess water quality and ecological balance.
Examples
Example 1: Calculate the pH of a 0.1 M solution of acetic acid (Ka = 1.8 x 10^-5).
Step 1: Ka = 1.8 x 10^-5
Step 2: Ka = [H+][A-] / [HA]
Step 3: 1.8 x 10^-5 = [H+][0.1] / [0.1]
Step 4: [H+] = 1.8 x 10^-5
Step 5: pH = -log(1.8 x 10^-5) = 4.74
Example 2: Determine the molarity of a solution of hydrochloric acid (HCl) with a pH of 2.5.
Step 1: pH = 2.5
Step 2: [H+] = 10^-pH = 10^-2.5
Step 3: Ka = [H+][A-] / [HA] for HCl, Ka is very large
Step 4: [HA] = [H+] = 10^-2.5
Step 5: Molarity = [HA] = 10^-2.5 M
Conclusion
Calculating pH from Ka and molarity provides a fundamental understanding of acid dissociation and its applications in various fields. By comprehending this relationship, researchers, scientists, and practitioners can accurately determine the pH of solutions, enabling them to optimize chemical reactions, assess biological systems, and monitor environmental conditions.