Can Ketones Participate in Hydrogen Bonding: A Detailed Examination
Introduction

Understanding intermolecular interactions is crucial in various scientific disciplines, including chemistry, biology, and materials science. Hydrogen bonding, a type of non-covalent interaction, plays a significant role in determining the properties and behavior of many compounds. Ketones, a class of organic compounds containing a carbonyl group (C=O), are known for their ability to participate in a variety of non-covalent interactions. However, the question of whether ketones can engage in hydrogen bonding with water remains a topic of debate. This article aims to provide a comprehensive overview of the current understanding of this intriguing phenomenon, exploring the experimental evidence, theoretical studies, and potential applications.
Ketones: An Overview
Ketones are characterized by the presence of a carbonyl group, which consists of a carbon atom double-bonded to an oxygen atom (C=O). They are commonly found in various natural products, such as carbohydrates, terpenes, and steroids. Ketones possess a polar carbonyl group, which is responsible for their solubility in polar solvents.
Hydrogen Bonding: A Primer
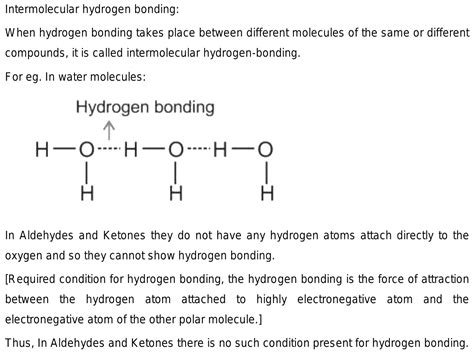
Hydrogen bonding is a non-covalent interaction that occurs between a hydrogen atom covalently bonded to an electronegative atom (such as oxygen, nitrogen, or fluorine) and another electronegative atom. The hydrogen atom is partially positive, while the electronegative atom is partially negative, creating a dipole-dipole interaction. Hydrogen bonds are weaker than covalent bonds but stronger than van der Waals forces, significantly influencing the physical and chemical properties of compounds.
Ketones and Hydrogen Bonding with Water: Experimental Evidence
Early experimental studies suggested that ketones do not participate in hydrogen bonding with water. However, more recent investigations using advanced techniques have challenged this notion. For instance, infrared (IR) spectroscopy studies have revealed that ketones exhibit a shift in their carbonyl stretching frequency in the presence of water, indicating the formation of hydrogen bonds. Additionally, nuclear magnetic resonance (NMR) spectroscopy has provided evidence for the existence of hydrogen-bonded complexes between ketones and water molecules.
Theoretical Studies: Unveiling the Molecular Interactions
Theoretical calculations based on quantum mechanics have shed further light on the nature of interactions between ketones and water. These studies have shown that hydrogen bonding between ketones and water is indeed possible, albeit weaker than the hydrogen bonding between water molecules themselves. The strength of the hydrogen bond depends on various factors, including the specific ketone structure, the solvent environment, and temperature.
Applications: Exploiting Hydrogen Bonding in Ketones
The understanding of hydrogen bonding between ketones and water has opened up new avenues for research and applications. For instance, the ability of ketones to form hydrogen bonds with water could be exploited in the design of novel materials with tailored properties. Additionally, this phenomenon could be utilized in the development of efficient water purification systems and the extraction of ketones from aqueous solutions.
Conclusion
The question of whether ketones can hydrogen bond with water has been a subject of ongoing scientific inquiry. Experimental evidence, supported by theoretical studies, has demonstrated that hydrogen bonding between ketones and water does occur, although it is weaker than the hydrogen bonding between water molecules themselves. The strength of the hydrogen bond depends on various factors, including the ketone structure, solvent environment, and temperature. Understanding this phenomenon has opened up new possibilities for applications in materials science, water purification, and extraction processes.